Abstract
Background
Cryoprecipitate (cryo) is a frozen plasma derivative consisting of fibrinogen, factor (F) XIII, FVIII and von Willebrand factor (vWF), and was originally intended to replace FVIII in hemophilia. Today, cryo is primarily used as fibrinogen replacement in acquired coagulopathy related to trauma, obstetric hemorrhage, and DIC. Due to the lability of FVIII, guidelines mandate that cryo be given within 6 hours of thawing. Fibrinogen is much more stable; however, and these time constraints may unnecessarily contribute to delayed product utilization and increased waste. Viscoelastic assays are increasingly used to assess coagulopathy and may be better indicators of global coagulation function than standard coagulation assays. Here we evaluated the performance of thawed, refrigerated cryoprecipitate over extended storage times.
Methods
Cryo (six donor pools, n=8) was purchased from South Texas Blood and Tissue, thawed on first day of testing, and stored in aliquots refrigerated at 4°C for up to 35 days. Assays were performed in 37°C rewarmed product at baseline post-thaw and at 4, 24, and 72h, and then weekly to 35 days. Assays for factor levels were conducted in cryo diluted 1:10 in PBS and using a hematology analyzer (Stago) for fibrinogen and FVIII or by ristocetin cofactor assay (RCo) for vWF (Siemens). Coagulation and thrombin generation of stored cryo was tested in ROTEM and Calibrated Automated Thrombogram (CAT) by mixing cryo with frozen aliquots of cryo-poor plasma. A massive transfusion protocol (MTP) was simulated by combining cryo with red cells, fresh frozen plasma, and fresh apheresis platelets (1:1:1:1 ratio); this simulation was tested for coagulation with ROTEM and for vWF-mediated fluorescent-labeled platelet adhesion to collagen in a microfluidics flow system (Bioflux).
Results
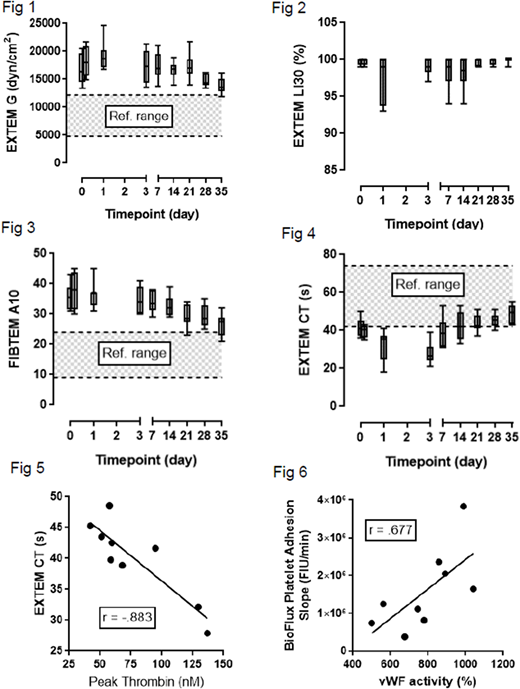
In simulated MTP, global coagulation function as measured by EXTEM and FIBTEM G-values from ROTEM were above normal reference ranges for whole blood for the entire 35d period (EXTEM smallest median G=13592 dyn/cm2, ref. range=4800-12250; FIBTEM smallest median G=2041, ref. range=495-1667). There was a slight decline in G value over storage duration, but it was not statistically significant (Fig 1). Lysis index at 30 min (LI30) in the EXTEM of simulated MTP did not statistically deviate over time (Fig 2).
The MTP FIBTEM A10 value, shown to be correlated with fibrinogen concentration, remains high over 35 days, with only some values on D35 dropping into the normal range (Fig 3; means at D0=35.75mm and D35=26.63; ref. range=9-24). A10 and G were highly correlated (r=.812, p=.008).
FVIII activity in cryo declined from 9-fold above average plasma levels over 35 days to approximately 25% of its starting activity. Surprisingly, the drop in FVIII was not correlated with clotting time (CT) in the EXTEM (r=-.335, p=.739), and CT was either faster than or within normal references ranges for whole blood at all time points (Fig 4; slowest median CT=49.5s, ref. range=42-72). The change in CT was correlated with measured peak thrombin levels (Fig 5; r=-.883, p=.002), although FVIII was not correlated with peak thrombin generation (r=.403, p=.282).
vWF activity of cryo initially increased as has been previously reported but declined to baseline level by D7 and further through D35. vWF activity was correlated with the rate of platelet adhesion to a collagen surface under arterial flow (Fig 6; r=.677, p=.045).
Discussion
Cryo is mainly used in resuscitation of severe hemorrhage in MTP. It contributes to hemostasis by providing vWF to support platelet adhesion and primary hemostasis, FVIII to augment thrombin generation, and fibrinogen as clot substrate. Standard coagulation assays such as PT and aPTT or isolated factor activity assays are inadequate for evaluating the contribution of cryo to global MTP performance. The data presented here, derived from multiple functional tests including ROTEM, CAT, ristocetin cofactor assay, and Bioflux, show that thawed cryoprecipitate stored at 4°C is functionally viable for up to 35 days; as part of a massive transfusion protocol. It supports clot strength, thrombin generation, and platelet adhesion. Adoption of an extended shelf life for thawed cryo would reduce waste and increase availability for treatment of hemorrhage.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.